orange book pharmacy definition
Food and Drug Administration in both bound and electronic formats. 2 approved over-the-counter OTC drug products for those drugs that.
Regulatory 101 Drug Name Modifiers Definition Categories Generics And Capa Ivt
The Orange Book is available on the FDAs Web site wwwfdagov under the Drugs link in the Spotlight section.

. Formally known as Approved Drug Products with Therapeutic Equivalence Evaluations the orange book lists drugs which are not only safe but also effective for human use. One prescription example would be combined oral contraception also. What does orange book mean.
The cost that the patient pays at the pharmacy point of sale. As mentioned the Orange Book serves as a guide for identifying suitable generic alternatives for branded products. National Association of Boards of Pharmacy.
The FDAs list of Approved Drug Products with Therapeutic Equivalence Evaluations. R i s k r e. Originally this book was published in October 1980 with orange cover and thus the name orange book.
Approved Drug Products with Therapeutic Equivalence Evaluations published by the FDAs Center for Drug Evaluation and Research. The Orange Book Risk Management Principles. Reclaiming Liberalism by members of the British Liberal Democrat party.
Looking for the definition of ORANGE BOOK. Rules and Guidance for Pharmaceutical Manufacturers and Distributors commonly known as the Orange Guide brings together all the main European and UK directives regulations and legislation relating to the manufacture and distribution of medicines. See note 5 supra.
Drug product selection laws. The FDA keeps a list known as the Orange Book of every approved therapeutic equivalent. Electronic Orange Book.
Compiled by the Inspection Enforcement and Standards Division Medicines and Healthcare. 1 approved prescription drug products with therapeutic equivalence evaluations. The orange book is a list of generic drugs approved by FDA.
Medical Dictionary 2009 Farlex and Partners. FDA orange book The official name of FDAs orange book is Approved Drug Products with Therapeutic Equivalence Evaluations. Get emails about this page.
Rucha Pathak Roll No. Information and translations of orange book in the most comprehensive dictionary definitions resource on the web. It is widely accepted as the authoritative source for determining therapeutic equivalence among multisource drug products.
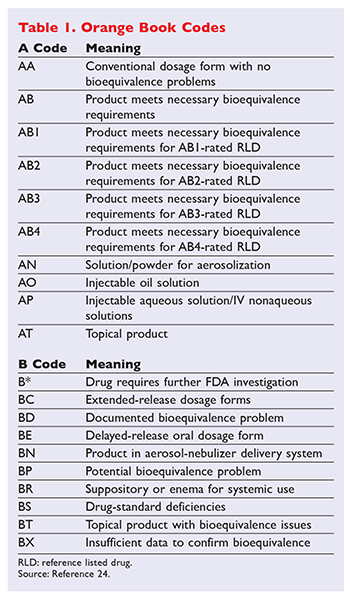
Before understanding different drug ratings it is necessary. A simple method of using the Orange Book for identifying therapeutically equivalent prescription medications. The Orange Book is composed of four parts.
The IUPAC Compendium of Analytical Nomenclature informally known as the Orange Book. FDAs Approved Drug Products with Therapeutic Equivalence Evaluations Orange Book identifies drug products approved on the basis of safety and effectiveness. Approved Drug Products with Therapeutic Equivalence Evaluations.
Therapeutic equivalence evaluations in this publication are not official FDA actions affecting the legal status of products under the Act See note 5 supra. Basics in drug approval process with reference to the Orange Book Presented by. An introduction a how to use section the drug product lists appendices and a patent and exclusivity information addendum.
Approved Drug Products with Therapeutic Equivalence Evaluations is one option -- get in to view more The Webs largest and most authoritative acronyms and abbreviations resource. See note 7 supra. Meaning of orange book.
The orange book consist of five main sections. G o v e r n a n c e and L e a d e r s I n te g ra o n h i p C o l a b or ti o n Information Insight Insight Information Communication. Survey of Pharmacy Laws.
The Orange book has been revised. The orange book is published annually and the 2015 edition is 35th edition of orange book1 It is freely available for. The Approved Drug Products with Therapeutic Equivalence Evaluations published by the US.
Typically refers to a drug product that can be purchased without a prescription. The Orange Book Introduction. The Orange Book formally titled Approved Drug Products With Therapeutic Equivalence Evaluations is a comprehensive list of approved drug products published by the FDA.
Updated with Orange Book.

The 2017 Orange And Green Guides Mhra Inspectorate

Introduction To Immunology Stomp On Step1 Immunology Innate Vs Adaptive Humoral Vs Cell Mediated
Orange Book And Its Applications Legal Advantage

Pharmacist Printable Definition Medical Office Or Wall Decor Professional Graduation Gift Minimal Pharmacy Quotes Pharmacist Aesthetic Words

Beeple Pharmacy Art Medical Artwork Iphone Wallpaper Hipster

Definition Of Heat Exhaustion Teamoarock Pay Attention Especially Those Of You Heading Out Racing Or Just On T Heat Exhaustion Throbbing Headache Exhaustion

The Apothecary Lee Dubin Metal Sign Etsy Pharmacy Art Apothecary Graphic Design Portfolio Inspiration

Vector Pharmacy And Open Book Logo Book Logo Professional Logo Design Open Book

The Orange Guide Medicinescomplete
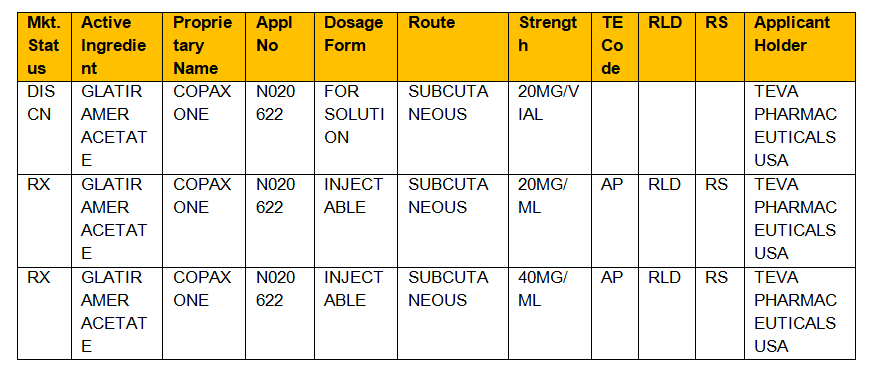
Insights Into Effective Generic Substitution

The Signs And Symptoms Of An Abdominal Aortic Aneurysm Aortic Aneurysm Abdominal Aortic Aneurysm Aneurysm

Have A Heart That Never Hardens Art Print Kindness Art Love Etsy Nurse Quotes Quotes Inspirational Words

Pin By Suzanne Peirsel On Art Lee Dubin Pharmacy Art Graphic Design Portfolio Inspiration Vintage Posters

Regenerative Medicine And Stem Cell Therapy For The Eye Ebook Rental Regenerative Medicine Stem Cell Therapy Stem Cells

List Of Branches Of Science And Definition Classification Of Science Branches Of Science Biology Facts Medical Knowledge

Scope In Pharmacy Pharmacy Hospital Pharmacy Medical Information
/doctor-826e0c116cd549d98e327f1184c622d9.jpg)